Efficacy of Elemental Sulfur Fertilizers

Elemental sulfur (S) is produced in large quantities in both the U.S. and Canada as a by‐product of fossil fuel production. However, this form of S must be oxidized to sulfate (SO42–) by soil microorganisms before crops can utilize it and therefore may not meet crop S requirements in the year of application. Rapid oxidation can be obtained if elemental sulfur particles are less than 20 μm in size and effectively dispersed in soil under favorable moisture and temperature conditions. Earn 0.5 CEUs in Nutrient Management by reading the article and taking the quiz at https://web.sciencesocieties.org/Learning‐Center/Courses.
The demand for sulfur (S) fertilizers has increased due to reduced S deposition and increased use of S‐demanding crops such as canola. Most fertilizers applied to alleviate S deficiencies are in the sulfate form, e.g., ammonium sulfate [(NH4)2SO4, 21‐0‐0‐24]. Sulfate can be directly taken up by plant roots.
Approximately 15 million tons of elemental S is recovered annually as a by‐product of fossil fuel production in North America. However, only a small fraction of this S is utilized directly as fertilizer for crops because this form must be oxidized by soil microorganisms before it is available to plants (Figure 1). If reliably oxidized, elemental S fertilizers provide advantages of high S content and low manufacturing cost.
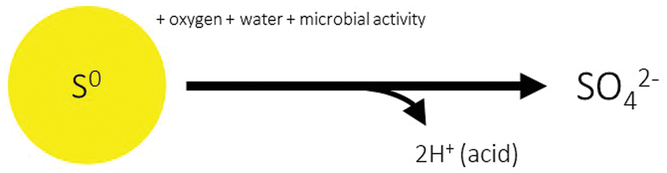
Factors Impacting Oxidation of Elemental Sulfur Fertilizers
Many different microorganisms capable of oxidizing elemental S are present in agricultural soils. Most elemental S oxidation is catalyzed by a diverse population of soil microorganisms rather than by specialized S oxidizers such as Thiobacillus oxidans (Germida & Janzen, 1993).
Elemental S oxidation occurs on the surface of elemental S particles, and therefore the rate of oxidation depends on the area, not quantity, of elemental S applied (Fox et al., 1964). Based on lab studies, the time required for 50% oxidation increased from one day to more than three years as particle diameter increased from 2 μm to 2 mm (Table 1). Particles must be less than 20 μm to significantly contribute to crop S requirements during the important early part of the growing season.
Per gram of elemental S | ||||
|---|---|---|---|---|
| Letter | Diameter (μm) | Number of particles | Surface area (cm2) | Days to oxidize 50%a |
| a | 2 | 1011 | 14,000 | 1 |
| b | 20 | 108 | 1,400 | 10 |
| c | 200 | 105 | 140 | 100 |
| d | 2,000 | 102 | 14 | 1000 |
aBased on Janzen & Bettany 1987 and Janzen, 1990.
First year | Second year | |||||
|---|---|---|---|---|---|---|
Trial location | Crop | SO4–S | ESa | Crop | SO4–S | ES |
Canada | Canola | 59 | 6 | Wheat | 7 | 13 |
Argentina | Corn | 78 | 12 | Soybean | 8 | 13 |
Brazil | Soybean/corn | 7 | 8 | Soybean/corn | 3 | 8 |
aES, elemental S, median diameter of 40 μm.
Elemental S oxidation is suppressed when particles are in close proximity to each other. This suppression may be due to water limitation caused by hydrophobicity and/or the accumulation of acidic or toxic oxidation products. Janzen (1990) found that oxidation was negligible when the soil‐to‐S ratio was less than 100:1 and increased rapidly as this ratio increased to 1,000:1. For this reason, banded application of elemental S fertilizer is less effective than broadcast application.
Elemental S is often co‐granulated with bentonite or other fertilizers to improve handling. Oxidation can be enhanced by reducing the proportion of elemental S and granule size in the final product. Compared with uniformly mixed particles, oxidation was 30‐fold slower for bentonite products (90% elemental S) but only fourfold slower for products co‐granulated with monoammonium phosphate (MAP) (5 to 7.5% elemental S) (Degryse et al., 2016).
The rate of elemental S oxidation also depends on environmental conditions. The process is more sensitive to temperature than most soil biological processes. Compared with oxidation at 86 °F (30 °C), oxidation was reduced 50% at 73 °F (23 °C) and 90% at 59 °F (15 °C) (Janzen & Bettany, 1987). The process is less sensitive to soil moisture, but as with many biological processes, the process is optimum at field capacity and reduced by dry or excessively wet conditions.
The ability of elemental S to meet crop requirements also depends on factors influencing the degree of S deficiency. Annual crops with a high and early S requirement (e.g., canola) will be more sensitive to delayed S supply than crops with a lower and later S requirement (e.g., corn). Crops grown on soils that are extremely deficient in S will be more sensitive to delayed S supply than crops grown on soils that are only moderately deficient in S.
Sulfur Source Comparisons
Three elemental S fertilizers were compared in central Alberta: a finely divided aqueous suspension (Micro‐S) and two bentonite products (Karamanos & Janzen, 1991). The products were applied at 0, 27, 54, and 108 lb S/ac. In the year of application, extractable soil SO4–S, canola S uptake, and canola seed yield were substantially increased by application of Micro‐S, at an efficacy equivalent to about 50% of ammonium sulfate. Benefits of bentonite products in the year of application were small to non‐existent as were residual benefits of all products in the two subsequent years.
A co‐granulated elemental S product (particles <10 μm, Sulvaris Inc., Calgary, AB) was compared with potassium sulfate on a S‐deficient site in Saskatchewan (Malhi et al., 2014). Both products were broadcast (fall and spring) and banded at 18 lb S/ac/yr in the same plots over a period of three years. Increases in S uptake of the co‐granulated product relative to potassium sulfate were 49% when broadcast and 21% when banded while increases in seed yield of hybrid canola were 84% when broadcast and 61% when banded. Subsequent testing in nine trials in the Midwest showed equal efficacy for corn yield and tissue S concentration for granular and liquid products manufactured with the same technology relative to ammonium sulfate (M. Howell, personal communication).
In a study conducted in Canada, Argentina, and Brazil, crop uptake of elemental S and sulfate from the same co‐granulated product (MES; The Mosaic Company, Tampa, FL) was determined by enriching each form independently with 34S (Degryse et al., 2020). Crop uptake of elemental S was considerably lower than that of SO4–S in both Canada and Argentina (Table 2), which was attributed primarily to low temperatures limiting oxidation of elemental S (median diameter of 40 μm). In contrast, crop recoveries of elemental S and SO4–S were similar but low in Brazil, which was attributed to warm and wet conditions that supported rapid oxidation and subsequent losses by leaching. Crop recovery of elemental S was higher than SO4–S in the second year, but cumulative recovery remained lower in Canada and Argentina.
The efficacy of a micronized elemental S fertilizer (Sulgro 70, manufactured by Sultech, Calgary, AB) sprayed on the soil surface without incorporation was compared with sprayed ammonium sulfate in eight field trials in southern and central Alberta over a two‐year period (Bremer et al., 2021). The supply of S to Plant Root Simulator (PRS) probes (ion‐exchange membranes) in soil was consistently increased by application of Sulgro 70 relative to the unamended control. On average, the increase in soil S supply of Sulgro 70 was 75% of that of ammonium sulfate between four and eight weeks after seeding for trials that received a minimum of 5 inches of rainfall. Based on increases in biomass S concentration in three trials where the unfertilized control had low S concentration (<3 g S kg–1), the relative uptake of Sulgro 70 was 34% compared with SO4–S. Canola seed yields were not increased by application of ammonium sulfate nor Sulgro 70 in any of the trials.
Conclusions
Elemental S fertilizers can be an effective and economical source of S for crops if particle size is less than about 20 μm, particles are effectively dispersed in soil, and moisture and temperature conditions are favorable for microbial activity. Earlier application or co‐application with sulfate may be necessary for adequate supply of S to cool‐season crops that have a high demand for S early in the growing season.
References
Bremer, E., Pauly, D., Strydhorst, S., & McKenzie, R. (2021). Evaluation of a sprayable elemental sulphur fertilizer under field conditions in Alberta. Canadian Journal of Soil Science, 101, 216–221. https://doi.org/10.1139/CJSS‐2020‐0134
Degryse, F., Ajiboye, B., Baird, R., da Silva, R.C., & McLaughlin, M.J. (2016). Oxidation of elemental sulfur in granular fertilizers depends on the soil‐exposed surface area. Soil Science Society of America Journal, 80, 294–305. https://doi.org/10.2136/sssaj2015.06.0237
Fox, R.L., Atesalp, H.M., Kampbell, D.H., & Rhoades, H.F. (1964). Factors influencing the availability of sulfur fertilizers to alfalfa and corn. Science Society of America Journal, 28, 406–408. https://doi.org/10.2136/sssaj1964.03615995002800030031x
Germida, J.J., & Janzen, H.H. (1993). Factors affecting the oxidation of elemental sulfur in soils. Fertilizer Research, 35, 101–114. https://doi.org/10.1007/BF00750224
Janzen, H.H., & Bettany, J.R. (1987). The effect of temperature and water potential on sulfur oxidation in soils. Soil Science, 144, 81–89. http://bit.ly/3EF5bQs
Janzen, H.H. (1990). Elemental sulfur oxidation as influenced by plant growth and degree of dispersion within soil. Canadian Journal of Soil Science, 70, 499–502. https://cdnsciencepub.com/doi/abs/10.4141/cjss90‐049
Karamanos, R.E., & Janzen, H.H. (1991). Crop response to elemental sulfur fertilizers in central Alberta. Canadian Journal of Soil Science, 71, 213–225. https://doi.org/10.4141/cjss91‐021
Malhi, S.S., Vera, C.L., & Brandt, S.A. (2014). Feasibility of a new granular rapid release elemental S fertilizer in preventing S deficiency of canola on a S‐deficient soil. Agricultural Sciences, 5. https://www.scirp.org/journal/paperinformation.aspx?paperid=50122
Self-Study CEU Quiz
Earn 0.5 CEUs in Nutrient Management by taking the quiz for the article at https://web.sciencesocieties.org/Learning-Center/Courses. For your convenience, the quiz is printed below. The CEU can be purchased individually, or you can access as part of your Online Classroom Subscription.
- Oxidation of elemental sulfur:
- is mostly catalyzed in agricultural soils by specialized microbial S oxidizers such as Thiobacillus oxidans.
- occurs on the surface of elemental S particles.
- only occurs under acidic conditions.
- is not required if the particle size of elemental S is less than 2 μm.
- Elemental sulfur with a particle size of 2 mm will be oxidized within:
- one week.
- one month.
- one year.
- more than 3 years.
- Oxidation of elemental S with fine particle size is most rapid when:
- applied as a liquid suspension and incorporated.
- applied as a co-granulated fertilizer in a band.
- applied as a liquid suspension in a band.
- broadcast as co-granulated fertilizer and incorporated.
- Rapid oxidation is most important when elemental S fertilizer is applied to meet S requirements for a:
- long-season crop with a low S demand on a soil with low S supply.
- long-season crop with a high S demand on a soil with moderate S supply.
- short-season crop with a high S demand on a soil with moderate S supply.
- short-season crop with a high S demand on a soil with low S supply.
- Elemental S fertilizers may be a better source of crop S than sulfate fertilizers:
- when used for long-season crops with a high S demand.
- when used in soils with very low soil organic matter.
- when elemental S oxidation is synchronized with crop S demand and soils have high leaching potential.
- under no circumstances.
Text © . The authors. CC BY-NC-ND 4.0. Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.










